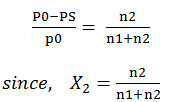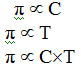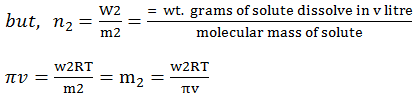SOLUTION
Solution: -
A solution is homogeneous mixture of two or more chemically non-reacting substances, whose composition can be varied within certain limits.Expressing concentration of solutions
1. Percentage:-
=> mass
percentage (%) of a component (W/W)
= Mass of the component in
solution*100 / Total mass of the
solution
=> volume
percentage (%) of the component (V/V)
= Volume of the component * 100 /
Total volume of the solution
=> Mass/Volume
percentage (%) of the component (W/V)
=Mass of the component in solution *
100 / Total volume of the solution
2. Strength:-
Strength of the solution ( gl-1
or gdm-3)
= Mass of the solute in grams /
Volume of the solution in litre
3. Molarity (M) :-
No. of moles of the solute / Volume of the solution in litre
M = strength in gram per litre / Molar mass of the solute
4. Mole fraction :-
It is obtained by dividing the no. of moles of the solute or solvent by the total no. of moles of the solution.
Mole fraction, X1
= n1/n1+n2
X2 = n2/n1+n2
n1 = no. of moles of the solvent
n2 = no. of moles of the solute
X1+X2 = 1
5. Mass fraction: -
Mass of the given component per unit mass of the solution denoted by X.
XA = WA / WA+WB
XB = WB / WA+WB
WA = Mass of the solvent
WB = Mass of the solute
XA+XB = 1
Solution of solids in liquids
Solubility of a solid in a liquid
:-
It is defined as the maximum amount of the solid (solute) in grams which can
dissolve in 100 grams of liquid (solvent) to form the saturated solution at the
particular temperature.
Factors affecting the solubility of a solid in a liquid
i.
Nature
of the solute & the solvent :- “Like dissolves Like”
=> The
polar (ionic) compounds like NaCl dissolve in polar solvent like water.
=> The
non polar (covalent or organic) compounds dissolve in non polar compounds like
Anthracene dissolve in Benzene.
ii.
Effect
of temperature :-
=> The
solubility increases with increase of temperature when the process of
dissolution is endothermic.
Solute
+ Solvent + Heat -> Solution
Ex:-
NaNo3, KNO3, NaCl, KCl
=> The
solubility Decreases with increase in temperature when the process of
dissolution is exothermic.
Solute
+ Solvent -> Solution + Heat
Ex:- Sodium carbonate mono
hydrate (Na2CO3.H2O).
=> Those
whose solubility does not increases or decreases continuously
Ex:-
CaCl2.6H2O -> CaCl2.4H2O ->
CaCl2.2H2O
Solution of Gases in liquids
1)
Solubility
of a gas in a liquid :- The solubility of a gas in a
particular liquid is the volume of the gas in CC’s (centimetre cubes CM3)
that can dissolve in unit volume of the liquid to form the saturated solution
at the temperature of the experimented under a pressure of one atmosphere.
Factors affecting the solubility of a liquid in a gas
i)
Nature
of the gas & the solvent:- Gases like Hydrogen,
Oxygen, Nitrogen etc. dissolve in water only to a small extent but gases like
CO2, HCl, NH3 are highly soluble.
The
greater solubility of later gases they react with solvent. the greater
solubility of the gas in a solvent due to their chemical similarity.
ii) Nature of temperature:- The
solubility of gases decreases with increase
in temperature.
iii) Effect of pressure (Henry’s Law):-
By increasing the pressure solubility also increases.
Henry’s Law:-
The mass of a gas dissolved in the given volume of liquid at constant temperature is directly proportional to the pressure of the gas present in equilibrium with the liquid.
m ∝
p
m=KHP
Also,
The partial pressure of gas in vapour phase (P) is
proportional to the mole fraction of gas (x) in the solution.
P=KHX
Limitations of Henry’s law
Henry’s law is applicable only if the the following
conditions are satisfied:-
a) Pressure
should be low and temperature should be high i.e. gas behaves as an ideal gas.
b) The
gas should not go under compound formation with solvent or association or
dissociation in the solvent.
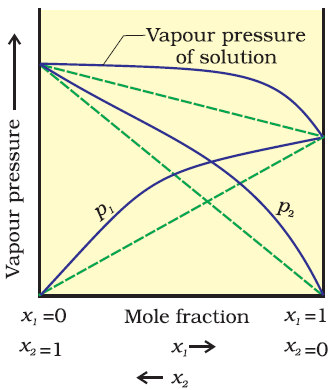
Vapour Pressure of liquid solution:-
Vapour pressure of liquid/solution is the pressure exerted by the vapour in equilibrium with the liquid/solution at a particular temperature.Raoults Law:-
It states that for a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction.
For component A
PA
∝ XA
PA
= P0AXA
Where, P0A =
Vapour pressure of pure component A at the same temperature
For component B,
PB
= P0BXB
By Daltons law of partial pressure:-
Ptotal
= PA+ PB
Ptotal
= P0AXA + P0BXB
= (1-XB) P0A+
P0BXB
= P0A - P0AXB
+ P0BXB
=
P0A+ (P0B- P0A)XB
If YA & YB represents the mole
fraction of component A & B respectively.
In vapour phase,
YA = PA/
PA+ PB = PA/ Ptotal ……………………..(i)
YB = PB/
PA+ PB = PB/ Ptotal ……………………..(ii)
From eq. (i) &
(ii)………
PA = YA
* Ptotal
PB = YB
* Ptotal
In general,
Pi = Yi * Ptotal
Raoults Law for non volatile liquid:-
Vapour pressure of each
component is directly proportional to its mole fraction.
P1 µ
X1
P1 = P01X1
P1/P01 = X1
Ideal solutions:-
An ideal is that solution which obeys
raoults law in all conditions of temperature an concentration.
i.e. ∆ Vmixing
= 0
∆ Hmixing = 0
Also, forces of
attraction between components are same as in pure state.
A-A = A-B = B-B
Non ideal solutions:-
The solutions which not obeys the
Raoults Law is known as non ideal solutions.
∆ Vmixing ≠
0, ∆ Hmixing ≠ 0
v The
force of interaction between solvent/solute is different than in pure state.
i.e. interaction between solute-solvent is weaker
than solute-solute or solvent-solvent. This means in such solutions the
molecules of A or B (A-B) will find it easier to escape than in pure state.
Negative
deviation:-

Negative Deviation
Intra molecular attraction force between
A-A and B-B are weaker than those between A-B. this decreases the escaping
tendency of molecules for each component (A-B) and consequently the vapour
pressure decreases resulting in –Ve deviation from raoults law.
Colligative Properties: -
Those properties of ideal solutions which depends only on the no. of particles of the solute dissolve in the definite amount of solvent and do not depend upon nature of solute.
The
important colligative properties are:-
1) Relative lowering of vapor pressure
2) Osmotic pressure
3) Elevation in boiling point
4) Depression in freezing point
Relative lowering of vapour pressure: -
from Raoults law for solutions of solids in liquids.
PS = vapour pressure of
solution
n2 = no. of moles of
solute
n1 = no. of moles of
solvent
For
dilute solutions n2<<n1
Here,
w1 & w2 are the masses and m1 & m2
are masses of solvent & solute respectively.Osmotic Pressure:-
The process of the flow of the solvent molecules from solvent to solution through semi permeable membrane (SPM) is called osmosis the flow of the solvent molecules can be stopped if some extra pressure is applied on the solution. This pressure that just stops the flow of solvent molecules is called osmotic pressure of the solution.
=>> Osmotic pressure
of the solution is directly proportional to the molar concentration or molarity
(C) of the solution and its temperature (T).
Mathematically,
Or, π = R*C*T
Where, R is solution constant equal to
the gas constant
π
= CRT
Hence, π = (n2/v) RT
this equation is called von’t hoff
equation for dilute solutions.
Elevation in boiling point:-
It is found that the boiling point of the solution is always higher than that of pure solvent. The increase is called elevation in boiling point.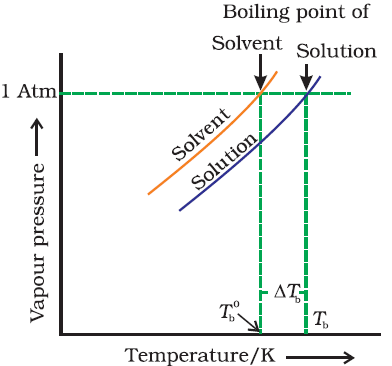 |
| Elevation in boiling point |
From
figure, we known ∆Tb
= Tb - T0b
Where,
T0b & Tb is
the temperature at which the vapour pressure of the solvent and solution
becomes equal to the atmospheric pressure and ∆Tb is called
elevation in boiling point.
Also,
∆Tb is directly proportional to m
∆Tb = kbm
Where,
kb is called molal elevation constant or ebullioscopy constant and
‘m’ is the molality of the solution.
=> Calculation of molecular mass of the solute:-
As molality is the no. of moles of the solute dissolve per 100 gm of the solvent. If w2 grams of the solute of molecular mass m2 are dissolve in w1 gram of the solvent
m = (W2/M2)(1000/W1)
Depression in freezing point:-
It is observed that the freezing point of the solution is always lower than that of pure solvent. And the decrease is called the depression in freezing point. |
| Depression in freezing point |
From figure, ∆Tf = T0f –Tf
Where, T0f = freezing point of
pure solvent
Tf = freezing point of solution
∆Tf = Depression in
freezing point
Also,
∆Tf is directly proportional to m
∆Tf = kf m
Where,
kf is molal depression constant or cryoscopic constant and m is
molality of the solution.
=> Calculation of molecular mass of solute:-
∆Tf =
Kf(w2/m2)(1000/W1)