General Principle and Process of Isolation of Elements
Minerals: -
These are naturally occurring chemical substances in the in the earth crust obtainable by mining.Ores : -
only few minerals are used as sources of that metal. Such minerals are known as ores.Gangue: -
ores are usually contaminated with earthly or undersides materials known as gangue.Metallurgy: -
The entire scientific and technological process used for isolation of the metal from its ores is known as metallurgy.Concentration of the ore: -
The removal of earthy and siliceous impurities (i.e. gangue or matrix) from the ores is called concentration of ores.(i) Hydraulic washing: -
The processes by which lighter earthy particles are free from the heavier ore particles by washing with water.(ii) Magnetic separation: -
This method of concentration is employed when either the ore or impurities associated with it are magnetic in nature.
Example:
- chromate.
(iii) Froth floatation: -
This method is based upon the fact that surface of sulphide ores is preferentially wetted by oils while that of gangue is preferentially wetted by water.(iv) Leaching: -
This process consists in treating the powdered ore with a suitable reagent which are selectively dissolved the ore but not the impurities.1) Leaching of Alumina from bauxite: -
ore of aluminum, bauxite usually contain SiO2, iron oxide, Tio2, as impurities .the concentration is carried out by digesting the powdered ore with the concentrated solution of NaOH at 473 to 523 K and 35-36 bar pressure. This way Al2O3 is leached out as sodium aluminates behind.
·
3H2O+Al2O3+2NaOH
----> 2Na[Al(OH)4]
The aluminates in solution is neutralized by passing
Co2 gas and hydrated Al2O3
is precipitated.
·
2Na[Al(OH)4]+CO2 ----> Al2O3*xH2O +
2NaHCO3
NaHCO3 remains
in the solution and hydrated alumina is filtered, dried and heated to give back
pure Al2O3.
2) Other Examples: -
In the metallurgy of silver and that of gold , the respective metal is leached with a dilute solution of NaCN or KCN in the presence of air (for O2) from which the metal is obtained later by replacement.
4[M(CN)2]- + Zn ----> [Zn(CN)4]2- + 2M
Extraction of crude metal from concentrated Ore :-
The concentrated are must be converted into a form
which is suitable for reduction. The process used to obtain metals in Free
State from concentrated ores is called extraction. It involves two chemical processes-
1. Conversion of the ore into metallic oxide or de-electronation of ores: -
Metal usually present as hydroxides, carbonates and sulphides depending upon the nature of the minerals present in the ore, the following two present in the ore, the following two methods are used for conversion of ore into their respective oxides.(a) Calcinations: -
It is the process of converting on ore into is oxide by heating it strongly below its melting point either in absent or limited supply of air during calcinations following change occur
(i)
Moisture is driven out
(ii)
Volatile impurities of S, As and P are removed as their volatile oxides
(b) Roasting: -
It is the process of converting an ore into its metallic oxide by heating strongly at temperature insufficient to melt in excess of air. This process is usually used for sulphide ores. Following change occur during roasting.
(i)
Moisture
is removed
(ii)
Organic matter is destroyed
(iii)
Non metallic impurities like that of S,
P and Ar are oxidized and removed as volatile gases.
S8
+ 8O2 ---->
8SO2 (Sulphur dioxide)
(Sulphur dioxide)
 (Sulphur dioxide)
(Sulphur dioxide)
P4
+ 5O2 ---->
P4O10 (Phosphorus pentaoxide)
(Phosphorus pentaoxide)
 (Phosphorus pentaoxide)
(Phosphorus pentaoxide)
4As
+ 3O2 ---->
2As2O3 (Arsenious oxide)
(Arsenious oxide)
 (Arsenious oxide)
(Arsenious oxide)
(iv)
Ores are generally converted into
metallic oxides
2ZnS
(Zinc sulphide) + 3O2 ----> 2ZnO
(Zinc oxide) + 2SO2

2PbS
(Lead oxide) + 3O2 ---->
2PbO (Lead oxide) + 2SO2

2Cu2S
(Cuprous sulphide) + 3O2 ----> 2Cu2O (Cuprous oxide) + 2SO2

Like
calcinations, roasting is also carried out in a reverberatory furnace
Thermodynamic principles of metallurgy: -
For any process, Gibbs free energy change (∆G) is given by
∆G = ∆H - T∆S
When
∆H is the enthalpy change and ∆S is the entropy change and T is the absolute change
for any reaction
∆Gϴ
= -RT ln K
Where,
k is the equilibrium constant of the reactant – product system at the
temperature T, A negative ∆G implies a +ve K in equation and this can happen
only when reaction proceeds towards product.
Applications:-
(a) Extraction of iron from its oxides -
Oxides ores of iron after concentration through calcinations/ roasting are mixed with limestone and coke and fed into a blast blast-furnace from its top. Here, the oxides is reduced to the metals
FeO + C ----> Fe + CO
And FeO is reduced and
C is oxidized to CO.
FeO
----> Fe + 1/2O2
C
+ 1/2O2 ----> CO
At 500-800 K
3Fe2O3
+ CO ----> 2Fe2O4
+ CO2
Fe3O4 + 4CO ----> 3Fe +
4CO2
Fe2O3 +CO ----> 2FeO + CO2
At 900-1500 K
C + CO2 ----> 2CO
FeO +CO ----> Fe +CO2
Limestone is also
decompose to CaO which remove silicate impurities of the ore as a slag
CaCO3 ----> CaO + CO2
CaO + SiO2 ---->
CaSiO3 (slag)
The iron obtained from
blast furnace contains about 4% carbon and many impurities in smaller amount (e.g.
S, P, Si, Mn) this is known as pig iron.
(b) Extraction of copper from cuprous oxide: -
Most of the ores are sulphide and some may also contain iron . The sulphide ores are roasted/ melted to give oxides.
2Cu2S + 3O2 ----> 2Cu2O + 2SO2
The oxide then can be
easily reduced to metallic copper using coke.
Cu2O + C ----> 2Cu + CO
And slag of iron
silicate is produced
FeO + SiO2 ---->
FeSiO3 (slag)
Following reaction also
takes place
2FeS + 3O2 ---->
2FeO + 2SO2
FeO + SiO2 ---->
FeSiO3
2Cu2S + 3O2 ----> 2Cu2O + 2SO2
2Cu2O + 2Cu2S
----> 6Cu + SO2
The
metal is distilled off and collected by rapid chilling
(d) Extraction of Aluminum: -
Alumina Al2O3 is mixed with Na3AlF6 or CaF2 which lowers the melting point of the mixture and brings conductivity. Fused mixture is electrolyzed. Steel cathode and graphite anode is used. The graphite anode is useful for the reduction of metal, the overall reaction are as follow-
2Al2O3
+ 3C ---->
4Al + 3CO2
The
electrolytic reactions are –
Cathode:
Al3+ + 3e- ----> Al
Anode:
C + O2- ----> CO + 2e-
C + 2O2- ----> CO2 + 4e-
(e) Extraction of copper low grad ores and scraps :-
Copper is extracted by hydro metallurgy from low grade ores it is leached out using acid or bacteria the sol containing Cu2+ is treated with scrape iron or hydrogen.
Cu2+
+ H2 ----> Cu + 2H+
Oxidation reduction: -
Besides reduction, some extraction are based on oxidation particularly for non-metals. For extraction of chlorine from brine.
2Cl-
+ 2H2O ---->
2OH- + H2 + Cl2
Refining: -
The process of purifying the crude metals is called refining. Some methods of refining are as follow.a) Distillation: -
This is very useful for low boiling metals like zinc and mercury. The impure metals are evaporated to obtain the pure metals as distillate.b) Liquation: -
In this method a low melting metal like tin can be made to flow on a sloping surface, in this way it is separated from higher melting impurities.c) Electrolytic refining: -
In this method the impure metal is made to act as anode a strip of some metal in pure form is used as cathode. They are put in a suitable electrolytic bath containing soluble salt of the some metals the more basic metal remains in the solution and the less basic ones go to the anode mud.
Anode:
M ----> Mn+
+ ne-
Cathode: Mn+ + ne- ----> M
d) Zone refining: -
In this method a circular mobile heater is fixed at one end of a rod or the impure metal, as heater more forward, the pure metal crystallises out of the melt and impurities pass on into the adjacent molten zone.e) Vapour phase refining :-
The crude metals is free from impurities by first converting it into a suitable volatile compound by heating it with specific reagent at a lower temperature and then decomposing the volatile compound at some higher temperature to give the pure metal.Ex: - (1) Mond Process and (2) van arkel method
f) Chromatographic method: -
The method is based upon the principle that the different components of a mixture are adsorbed to different extents of an adsorbent.
For example in column chromatography,
different components of the mixture are adsorbed to different extents depending
upon their polarity.


![Minerals: - These are naturally occurring chemical substances in the in the earth crust obtainable by mining. Ores : - only few minerals are used as sources of that metal. Such minerals are known as ores. Gangue: - ores are usually contaminated with earthly or undersides materials known as gangue. Metallurgy: - The entire scientific and technological process used for isolation of the metal from its ores is known as metallurgy. Concentration of the ore: - the removal of earthy and siliceous impurities (i.e. gangue or matrix) from the ores is called concentration of ores. Hydraulic washing: - The processes by which lighter earthy particles are free from the heavier ore particles by washing with water. Magnetic separation: - This method of concentration is employed when either the ore or impurities associated with it are magnetic in nature. Example: - chromate. Froth floatation: - This method is based upon the fact that surface of sulphide ores is preferentially wetted by oils while that of gangue is preferentially wetted by water. Leaching: - this process consists in treating the powdered ore with a suitable reagent which are selectively dissolved the ore but not the impurities. Leaching of Alumina from bauxite: - ore of aluminium, bauxite usually contain SiO2, iron oxide, Tio2, as impurities .the concentration is carried out by digesting the powdered ore with the concentrated solution of NaOH at 473 to 523 K and 35-36 bar pressure. This way Al2O3 is leached out as sodium aluminates behind. 3H2O+Al2O3+2NaOH 2Na[Al(OH)4] The aluminates in solution is neutralised by passing Co2 gas and hydrated Al2O3 is precipitated. 2Na[Al(OH)4]+CO2 Al2O3*xH2O + 2NaHCO3 NaHCO3 remains in the solution and hydrated alumina is filtered, dried and heated to give back pure Al2O3. Al_2 O_3.xH_2 O □(→┴( ∆ ) Al_2 O_3 + xH_2 O) Other Examples: - In the metallurgy of silver and that of gold , the respective metal is leached with a dilute solution of NaCN or KCN in the presence of air (for O2) from which the metal is obtained later by replacement. 4M + 8CN^- + 2H_2 O + O_2 □(→┴( ∆ ) 4[M(CN)_2 ]^-+ 4OH^- ) Where M = Ag or Au. 4[M(CN)2]- + Zn [Zn(CN)4]2- + 2M Extraction of crude metal from concentrated are :- The concentrated are must be converted into a form which is suitable for reduction. The process used to obtain metals in Free State from concentrated ores is called extraction. It involves two chemical processes- Conversion of the ore into metallic oxide or de-electronation of ores: - Metal usually present as hydroxides, carbonates and sulphides depending upon the nature of the minerals present in the ore, the following two present in the ore, the following two methods are used for conversion of ore into their respective oxides. Calcinations: - it is the process of converting on ore into is oxide by heating it strongly below its melting point either in absent or limited supply of air during calcinations following change occur Moisture is driven out Volatile impurities of S, As and P are removed as their volatile oxides Water is remove from hydrated oxides and hydroxide ores Al_2 O_3.2H_2 O(Bauxite) □(→┴( ∆ ) ) Al_2 O_3 (Alumina) + 2H_2 O Carbonate ore are converted into their respective oxides by lose of carbon dioxide CaCO_3 (Lime Stone) □(→┴( ∆ ) ) CaO (Calcium Oxide) + CO_2 Roasting: - it is the process of converting an ore into its metallic oxide by heating strongly at temperature insufficient to melt in excess of air. This process is usually used for sulphide ores. Following change occur during roasting. Moisture is removed Organic matter is destroyed Non metallic impurities like that of S, P and Ar are oxidised and removed as volatile gases. S8 + 8O2 8SO2 (Sulphur dioxide) P4 + 5O2 P4O10 (Phosphorus pentaoxide) 4As + 3O2 2As2O3 (Arsenious oxide) Ores are generally converted into metallic oxides 2ZnS (Zinc sulphide) + 3O2 2ZnO (Zinc oxide) + 2SO2 2PbS (Lead oxide) + 3O2 2PbO (Lead oxide) + 2SO2 2Cu2S (Cuprous sulphide) + 3O2 2Cu2O (Cuprous oxide) + 2SO2 Like calcinations, roasting is also carried out in a reverberatory furnace Thermodynamic principles of metallurgy: - for any process, Gibbs free energy change (∆G) is given by ∆G = ∆H - T∆S When ∆H is the enthalpy change and ∆S is the entropy change and T is the absolute change for any reaction ∆Gϴ = -RT ln K Where, k is the equilibrium constant of the reactant – product system at the temperature T, A negative ∆G implies a +ve K in equation and this can happen only when reaction proceeds towards product. Applications:- Extraction of iron from its oxides - oxides ores of iron after concentration through calcinations/ roasting are mixed with limestone and coke and fed into a blast blast-furnace from its top. Here, the oxides is reduced to the metals FeO + C Fe + CO And FeO is reduced and C is oxidised to CO. FeO Fe + 1/2O2 C + 1/2O2 CO At 500-800 K 3Fe2O3 + CO 2Fe2O4 + CO2 Fe3O4 + 4CO 3Fe + 4CO2 Fe2O3 +CO 2FeO + CO2 At 900-1500 K C + CO2 2CO FeO +CO Fe +CO2 Limestone is also decompose to CaO which remove silicate impurities of the ore as a slag CaCO3 CaO + CO2 CaO + SiO2 CaSiO3 (slag) The iron obtained from blast furnace contains about 4% carbon and many impurities in smaller amount (e.g. S, P, Si, Mn) this is known as pig iron. Extraction of copper from cuprous oxide: - Most of the ores are sulphide and some may also contain iron .the sulphide ores are roasted/ melted to give oxides. 2Cu2S + 3O2 2Cu2O + 2SO2 The oxide then can be easily reduced to metallic copper using coke. Cu2O + C 2Cu + CO And slag of iron silicate is produced FeO + SiO2 FeSiO3 (slag) Following reaction also takes place 2FeS + 3O2 2FeO + 2SO2 FeO + SiO2 FeSiO3 2Cu2S + 3O2 2Cu2O + 2SO2 2Cu2O + 2Cu2S 6Cu + SO2 Extraction of zinc from zinc oxide: - The reduction of zinc oxide is done by using coke ZnO + C □(→┴( coke, 673K ) Zn + CO) The metal is distilled off and collected by rapid chilling Extraction of Aluminium: - Alumina Al2O3 is mixed with Na3AlF6 or CaF2 which lowers the melting point of the mixture and brings conductivity. Fused mixture is electrolysed. Steel cathode and graphite anode is used. The graphite anode is useful for the reduction of metal, the overall reaction are as follow- 2Al2O3 + 3C 4Al + 3CO2 The electrolytic reactions are – Cathode: Al3+ + 3e- Al Anode: C + O2- CO + 2e- C + 2O2- CO2 + 4e- Extraction of copper low grad ores and scraps :- Copper is extracted by hydrometallurgy from low grade ores it is leached out using acid or bacteria the sol containing Cu2+ is treated with scrape iron or hydrogen. Cu2+ + H2 Cu + 2H+ Oxidation reduction: - besides reduction, some extraction are based on oxidation particularly for non-metals. For extraction of chlorine from brine. 2Cl- + 2H2O 2OH- + H2 + Cl2 Refining: - The process of purifying the crude metals is called refining. Some methods of refining are as follow. Distillation: - This is very useful for low boiling metals like zinc and mercury. The impure metals are evaporated to obtain the pure metals as distillate. Liquation: - In this method a low melting metal like tin can be made to flow on a sloping surface, in this way it is separated from higher melting impurities. Electrolytic refining: - in this method the impure metal is made to act as anode a strip of some metal in pure form is used as cathode. They are put in a suitable electrolytic bath containing soluble salt of the some metals the more basic metal remains in the solution and the less basic ones go to the anode mud. Anode: M Mn+ + ne- Cathode: Mn+ + ne- M Zone refining: - In this method a circular mobile heater is fixed at one end of a rod or the impure metal, as heater more forward, the pure metal crystallises out of the melt and impurities pass on into the adjacent molten zone. Vapour phase refining :- The crude metals is free from impurities by first converting it into a suitable volatile compound by heating it with specific reagent at a lower temperature and then decomposing the volatile compound at some higher temperature to give the pure metal. Ex: - (1) Mond Process □(Ni + 4CO →┴( 330-350K ) ) Ni(CO)_4 (nickel tetra carbonyl) Impure nickel Ni(CO)_4 →┴( 450-470K ) Ni (pure nickel)+ 4CO (2) van arkel method Zr(S)+ 2I_2 (g) →┴( 870K ) ZrI_4 (g) Impure zirconium ZrI_4 →┴( 2075K & tungusten filament ) Zr(S) (pure zirconium) + 2I_2 (g) Chromatographic method: - The method is based upon the principle that the different components of a mixture are adsorbed to different extents of an adsorbent. For example in column chromatography, different components of the mixture are adsorbed to different extents depending upon their polarity. Minerals: - These are naturally occurring chemical substances in the in the earth crust obtainable by mining. Ores : - only few minerals are used as sources of that metal. Such minerals are known as ores. Gangue: - ores are usually contaminated with earthly or undersides materials known as gangue. Metallurgy: - The entire scientific and technological process used for isolation of the metal from its ores is known as metallurgy. Concentration of the ore: - the removal of earthy and siliceous impurities (i.e. gangue or matrix) from the ores is called concentration of ores. Hydraulic washing: - The processes by which lighter earthy particles are free from the heavier ore particles by washing with water. Magnetic separation: - This method of concentration is employed when either the ore or impurities associated with it are magnetic in nature. Example: - chromate. Froth floatation: - This method is based upon the fact that surface of sulphide ores is preferentially wetted by oils while that of gangue is preferentially wetted by water. Leaching: - this process consists in treating the powdered ore with a suitable reagent which are selectively dissolved the ore but not the impurities. Leaching of Alumina from bauxite: - ore of aluminium, bauxite usually contain SiO2, iron oxide, Tio2, as impurities .the concentration is carried out by digesting the powdered ore with the concentrated solution of NaOH at 473 to 523 K and 35-36 bar pressure. This way Al2O3 is leached out as sodium aluminates behind. 3H2O+Al2O3+2NaOH 2Na[Al(OH)4] The aluminates in solution is neutralised by passing Co2 gas and hydrated Al2O3 is precipitated. 2Na[Al(OH)4]+CO2 Al2O3*xH2O + 2NaHCO3 NaHCO3 remains in the solution and hydrated alumina is filtered, dried and heated to give back pure Al2O3. Al_2 O_3.xH_2 O □(→┴( ∆ ) Al_2 O_3 + xH_2 O) Other Examples: - In the metallurgy of silver and that of gold , the respective metal is leached with a dilute solution of NaCN or KCN in the presence of air (for O2) from which the metal is obtained later by replacement. 4M + 8CN^- + 2H_2 O + O_2 □(→┴( ∆ ) 4[M(CN)_2 ]^-+ 4OH^- ) Where M = Ag or Au. 4[M(CN)2]- + Zn [Zn(CN)4]2- + 2M Extraction of crude metal from concentrated are :- The concentrated are must be converted into a form which is suitable for reduction. The process used to obtain metals in Free State from concentrated ores is called extraction. It involves two chemical processes- Conversion of the ore into metallic oxide or de-electronation of ores: - Metal usually present as hydroxides, carbonates and sulphides depending upon the nature of the minerals present in the ore, the following two present in the ore, the following two methods are used for conversion of ore into their respective oxides. Calcinations: - it is the process of converting on ore into is oxide by heating it strongly below its melting point either in absent or limited supply of air during calcinations following change occur Moisture is driven out Volatile impurities of S, As and P are removed as their volatile oxides Water is remove from hydrated oxides and hydroxide ores Al_2 O_3.2H_2 O(Bauxite) □(→┴( ∆ ) ) Al_2 O_3 (Alumina) + 2H_2 O Carbonate ore are converted into their respective oxides by lose of carbon dioxide CaCO_3 (Lime Stone) □(→┴( ∆ ) ) CaO (Calcium Oxide) + CO_2 Roasting: - it is the process of converting an ore into its metallic oxide by heating strongly at temperature insufficient to melt in excess of air. This process is usually used for sulphide ores. Following change occur during roasting. Moisture is removed Organic matter is destroyed Non metallic impurities like that of S, P and Ar are oxidised and removed as volatile gases. S8 + 8O2 8SO2 (Sulphur dioxide) P4 + 5O2 P4O10 (Phosphorus pentaoxide) 4As + 3O2 2As2O3 (Arsenious oxide) Ores are generally converted into metallic oxides 2ZnS (Zinc sulphide) + 3O2 2ZnO (Zinc oxide) + 2SO2 2PbS (Lead oxide) + 3O2 2PbO (Lead oxide) + 2SO2 2Cu2S (Cuprous sulphide) + 3O2 2Cu2O (Cuprous oxide) + 2SO2 Like calcinations, roasting is also carried out in a reverberatory furnace Thermodynamic principles of metallurgy: - for any process, Gibbs free energy change (∆G) is given by ∆G = ∆H - T∆S When ∆H is the enthalpy change and ∆S is the entropy change and T is the absolute change for any reaction ∆Gϴ = -RT ln K Where, k is the equilibrium constant of the reactant – product system at the temperature T, A negative ∆G implies a +ve K in equation and this can happen only when reaction proceeds towards product. Applications:- Extraction of iron from its oxides - oxides ores of iron after concentration through calcinations/ roasting are mixed with limestone and coke and fed into a blast blast-furnace from its top. Here, the oxides is reduced to the metals FeO + C Fe + CO And FeO is reduced and C is oxidised to CO. FeO Fe + 1/2O2 C + 1/2O2 CO At 500-800 K 3Fe2O3 + CO 2Fe2O4 + CO2 Fe3O4 + 4CO 3Fe + 4CO2 Fe2O3 +CO 2FeO + CO2 At 900-1500 K C + CO2 2CO FeO +CO Fe +CO2 Limestone is also decompose to CaO which remove silicate impurities of the ore as a slag CaCO3 CaO + CO2 CaO + SiO2 CaSiO3 (slag) The iron obtained from blast furnace contains about 4% carbon and many impurities in smaller amount (e.g. S, P, Si, Mn) this is known as pig iron. Extraction of copper from cuprous oxide: - Most of the ores are sulphide and some may also contain iron .the sulphide ores are roasted/ melted to give oxides. 2Cu2S + 3O2 2Cu2O + 2SO2 The oxide then can be easily reduced to metallic copper using coke. Cu2O + C 2Cu + CO And slag of iron silicate is produced FeO + SiO2 FeSiO3 (slag) Following reaction also takes place 2FeS + 3O2 2FeO + 2SO2 FeO + SiO2 FeSiO3 2Cu2S + 3O2 2Cu2O + 2SO2 2Cu2O + 2Cu2S 6Cu + SO2 Extraction of zinc from zinc oxide: - The reduction of zinc oxide is done by using coke ZnO + C □(→┴( coke, 673K ) Zn + CO) The metal is distilled off and collected by rapid chilling Extraction of Aluminium: - Alumina Al2O3 is mixed with Na3AlF6 or CaF2 which lowers the melting point of the mixture and brings conductivity. Fused mixture is electrolysed. Steel cathode and graphite anode is used. The graphite anode is useful for the reduction of metal, the overall reaction are as follow- 2Al2O3 + 3C 4Al + 3CO2 The electrolytic reactions are – Cathode: Al3+ + 3e- Al Anode: C + O2- CO + 2e- C + 2O2- CO2 + 4e- Extraction of copper low grad ores and scraps :- Copper is extracted by hydrometallurgy from low grade ores it is leached out using acid or bacteria the sol containing Cu2+ is treated with scrape iron or hydrogen. Cu2+ + H2 Cu + 2H+ Oxidation reduction: - besides reduction, some extraction are based on oxidation particularly for non-metals. For extraction of chlorine from brine. 2Cl- + 2H2O 2OH- + H2 + Cl2 Refining: - The process of purifying the crude metals is called refining. Some methods of refining are as follow. Distillation: - This is very useful for low boiling metals like zinc and mercury. The impure metals are evaporated to obtain the pure metals as distillate. Liquation: - In this method a low melting metal like tin can be made to flow on a sloping surface, in this way it is separated from higher melting impurities. Electrolytic refining: - in this method the impure metal is made to act as anode a strip of some metal in pure form is used as cathode. They are put in a suitable electrolytic bath containing soluble salt of the some metals the more basic metal remains in the solution and the less basic ones go to the anode mud. Anode: M Mn+ + ne- Cathode: Mn+ + ne- M Zone refining: - In this method a circular mobile heater is fixed at one end of a rod or the impure metal, as heater more forward, the pure metal crystallises out of the melt and impurities pass on into the adjacent molten zone. Vapour phase refining :- The crude metals is free from impurities by first converting it into a suitable volatile compound by heating it with specific reagent at a lower temperature and then decomposing the volatile compound at some higher temperature to give the pure metal. Ex: - (1) Mond Process □(Ni + 4CO →┴( 330-350K ) ) Ni(CO)_4 (nickel tetra carbonyl) Impure nickel Ni(CO)_4 →┴( 450-470K ) Ni (pure nickel)+ 4CO (2) van arkel method Zr(S)+ 2I_2 (g) →┴( 870K ) ZrI_4 (g) Impure zirconium ZrI_4 →┴( 2075K & tungusten filament ) Zr(S) (pure zirconium) + 2I_2 (g) Chromatographic method: - The method is based upon the principle that the different components of a mixture are adsorbed to different extents of an adsorbent. For example in column chromatography, different components of the mixture are adsorbed to different extents depending upon their polarity.](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjt-YG8miwsHwA06HSPw4XP1Ii4gr5X2V8aIwYn8pwja0UQLf7fs_JxZrtWsYF2M5XlwSdUkBPtzNgRmq1UeJXvZJvOlWSFX7I8yqMb13cfgg4UIFk36TVzY1BYYqHp9qtoczBDE3sKKGnY/s1600-rw/CHEMISTRY.png)
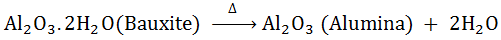


%2BMond%2BProcess%2Band%2B(2)%2B%2Bvan%2Barkel%2Bmethod.png)