Atomic Structure
In this chemistry notes post, we learn about Atomic Structure. These atomic structure notes are good for Graduation BSc and Post graduation MSc students.
Atomic Structure in Chemistry
Atom:
In Greek Atom means not divided. In 1803 john Dalton made a famous theory atomic theory according to this theory “matter is made up of tiny particles called atoms.” But in 1897 Sir J.J. Thomson proved experimentally that atoms are made of charged particles called electrons. And in the beginning of 20th century many scientists like Lord Rutherford, E. Goldstein proved that atom is not a smallest particle but is made up of electron, protons and neutrons.
Philosopher Scientists and their Ideas about Matter & Atom
1. Democritus
Ancient Greek Ideas About Matter
| |
Philosopher
|
Ideas
|
Democritus (460-370 B.C.)
Figure 1
|
· The ancient philosopher Democritus (ca 460–370 BC) was one of the first to propose that matter is constructed of tiny indivisible particles known as atomos (or atoms).
· Democritus also proposed that matter vary in shape and size depending on the substance they compose.
· According to Democritus, atoms could not be created nor destroyed.
|
2. Aristotle
Ancient Greek Ideas About Matter
| |
Philosopher
|
Ideas
|
Aristotle (384-322 B.C.)
Figure 2
|
· Democritus’ ideas about atoms were opposed by more popular philosophers, Aristotle (384–322 B.C.).
· He opposed the idea that atoms moved through empty space.
· According to Aristole, matter was made of four elements namely:
1. Earth
2. Wind
3. Water
4. Fire
|
3. John Dalton
- In the nineteenth century John Dalton (1766–1844), marks the beginning of the progress of modern atomic theory.
- John Dalton challenges the Aristotelian theory. Dalton revived and revised Democritus’s ideas based on the results of scientific research he conducted.
- The ideas of Democritus’s and Dalton’s were similar. Dalton carried out a number of experiments that allowed him to refine and support his hypotheses.
- Dalton carried out numerous chemicals reactions where he was able to determine the mass ratios of different elements involves in the chemical reactions.
- The outcomes of his research are known as Dalton’s atomic theory, which he proposed in 1803.
- Based on Dalton’s atomic theory, John Dalton calculated the first relative weights of atoms.
- He assessed the atomic weights of some elements according to the mass ratios in which they combined; with the hydrogen atom taken as unity.
Figure 3
These are atomic structure pdf for bsc 1st year notes by chemistrynotesinfo.com .
Dalton’s Atomic Theory
Dalton’s Atomic Theory
| |
Scientist
|
Ideas
|
Dalton (1766-1844)
Figure 4
|
1. Elements consist of extremely small particles called atoms.
2. All elements of a given element are identical, having the same size, mass and chemical properties.
3. Compounds are composed of more than one element. In any compound the number of atoms of any two of the elements present is either an integer or a simple fraction.
4. In a chemical reaction, atoms are separated, combined or rearranged.
|
Postulates of the Atomic Theory
Dalton expressed his theory in a series of postulates from structure of atom. He integrated the ideas of others into his own.
- Matter consists of atoms, which cannot be divided into simpler element. Therefore atoms can neither be created nor destroyed.
- Atoms of one element cannot be transformed into other atoms of an element. In chemical reactions, the atoms of an element can combine with same or other atoms of an element to form new substances.
- Atoms of an element have mass, physical and chemical properties and are different from atoms of any other element.
- Compounds are formed from a chemical reaction of a specific ratio of atoms of different elements.
How the Theory Explains the Mass Laws
Mass conservation
According to postulate 1 and 2, atoms cannot be created, destroyed and converted into other types of atoms. This means that in a chemical reaction, in which different atoms of an element are combined together cannot result in a mass change.
Definite composition
According to postulate 3 and 4, a compound composed of a specific ratio of different atoms, each of which has a particular mass. Therefore, each element in a compound contributes to a fixed fraction of the total mass.
Multiple proportions
According to postulate 1 and 3, atoms of an element have the same mass and are invisible. For instance, the number of atoms of any two of the elements present is either an integer or a simple fraction.
Nuclear Atom Model
The electron (Discovery of the Electron and Its Properties)
- In 1855, Sir William Crookes carried out a series on experiment to investigate the behavior of heated metals in a vacuum. The experiments showed that a heated cathode produced radiation, which could make substances to emit light.
- The radiation emitted from the cathode is called cathode rays.
- According to some research, it was known that cathode rays could be deflected by a magnetic field and electric field, and they carried a negative charge (the cathode is the negatively charged electrode and because the beam originated from the cathode, it must therefore be negatively charged).
- Subscribe Chemistry Notes Info for more chemistry notes related to atomic structure and follow us on Facebook Page of ChemistryNotesInfo.
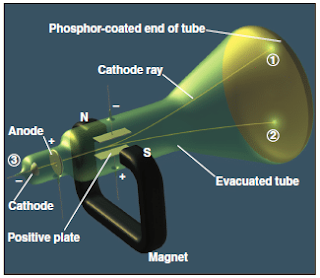
The cathode-ray tube
- In 1897, Joseph J. Thomson demonstrated that both the beam and the charged particles could be bent by an electrical field is applied perpendicular to the path of the beam, as shown in Figure 5.
- Thomson used the cathode-ray tube to show the deflection of electron by an applied electric field.
- When the cathode is heated, cathode rays is produced which travel along the tube and hit the phosphor-coated end of the tube and emit a glowing spot of light (Figure 5).
- The rays produced at the negative electrode (cathode) and moved to the positive electrode (anode).
- It was concluded that cathode rays consist of negatively charged particles found in all matter.
- By varying the electric field strength and measuring the angle of deflection, Thomson was able to determine the charge-to-mass (e/m) ratio of the particles, which are known as electrons.
- Thomson measured the e/m ratio as −1.76 × 108 C/g.
- According to Thomson, the e/m ratio is larger than the one expected compared to the atomic weights of the lightest of atoms. Therefore this indicates that the negatively charged electrons must be much smaller in size than a typical atom.
- As a result of his experiment, Thomson proposed the plum pudding model of the atom, where the atom consisted of one or more of these tiny electrons distributed in a sea of positive charge.
Figure 5 : The cathode-ray tube – Atomic Structure BSc 1st Year Chemistry Notes
Millikan’s oil-drop experiment for measuring an electron’s charge
Mass and Charge of the Electron
Two classic experiments revealed the mass and charge of the electron:
Mass/charge ratio
- In 1897, the British physicist J. J. Thomson (1856–1940) measured the ratio of the mass of a cathode ray particle to its charge.
- By comparing this value with the mass/charge ratio for the lightest charged particle in solution, he estimated that the cathode ray particle weighed less than as much as hydrogen, the lightest atom.
Charge
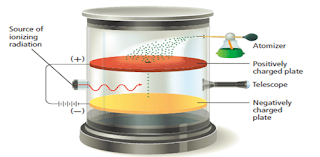
- In 1909, the American researcher Robert Millikan designed an experiment that measured the charge on the electron.
- He did so by observing the movement of oil droplets in an apparatus that contained electrically charged plates and an x-ray source as shown in Figure 6.
The motion of the oil droplets within Millikan’s apparatus depends on the charge of droplets and on the electric field. Millikan observed the droplets with the telescope. He could make the droplets fall more slowly, rise, or pause as he varied the strength of the electric field. From his observations, he calculated the charge on each droplet.
- This experiment also enabled the calculation of the mass of the electron based from its mass: charge ration obtained from J.J Thomson experiment.
- Millikan measured the charge on a great number of tiny oil drops which had been charged.
- An x-ray radiation is used to remove electrons from the gas molecules present in the chamber.
- The oil drops pick up the electrons present in the chamber, making them to become negatively charged.
- A small number of the oil drops sprayed into the box above the positively charged plate pass through the hole.
- When there is no electric field between the plates, the drops fall slowly with a steady velocity.
- An individual drop carrying a charge may be brought to rest by applying a voltage across the plates so that its weight ( acting downwards) is exactly balanced by the electrostatic force in it ( acting upwards).
- Millikan varies the voltage so that the oil droplet suspend in the air.
- Then he measured it total charge, by measuring the voltage needed to bring the drop to rest and the rate at which it falls when there is no voltage between the plates.
- Millikan was able to determine that each of the charged particles was some integral multiple of the electronic charge, which he determined to be −1.592 × 10−19 C, a measurement that is fairly close to the modern value for the charge on an electron (−1.60217733 × 10−19 C).
- As a drop could only pick up only whole number of electrons, this indicated that the charge on an individual electron must be -1.6 x 10-19.
- Therefore the value of electron’s charge is -1.602×10-19 C (C stands for coulomb, the SI unit of charge).
- Therefore the mass of the electron me could be calculated using the electron’s mass; charge ration and the value for the electron’s charge.
e/me = -1.76 x 1011 Ckg-1
me = e/(-1.76 x 1011)
me = (-1.6 x 10-19)/ (-1.76 x 1011)
me = 9.1 x 10-31 kg
The plum pudding model
- Thomson proposed the plum pudding model of the atom, where the atom consisted of one or more of these tiny electrons distributed in a sea of positive charge.
- Thomson thought that the positive charge necessary to counterbalance the negative charges of electrons in a neutral atom was in the form of a cloud.
- According to this model, an atom is spherical in shape and it consist of positive and negative charges equally distributed as shown in Figure 7.
Figure 7
The Nucleus of Atomic Structure
- In 1911, Ernest Rutherford (1871–1937) began to study how positively charged alpha particles interacted with solid matter.
- Rutherford carried out an experiment using a thin gold metal sheet to see if alpha particles would be deflected as they passed through the sheet.
Rutherford’s experiment
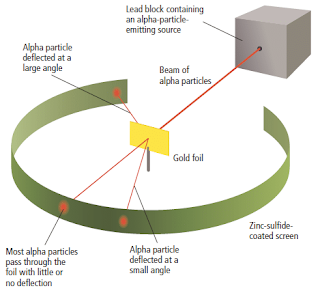
- The Rutherford’s gold fold experiment consists of a source of alpha particles, a thin gold sheet and a fluorescent screen. The screen consists of a phosphorescent coating of zinc-sulphide on its interior surface. The screen is circular in shape. The metal foil was mounted at the centre of the apparatus as shown in Figure 10.
- When an energetic alpha particle struck the phosphorescent screen, a flash of light would be observed. By noting where the flashes occurred, the scientists could determine if the atoms in the gold foil deflected the alpha particles.
- Based on the plum pudding model, where the electrons were evenly dispersed in a sphere of positive charge, Rutherford expected that the heavier alpha particles should be able to pass through the atom with little or no deflections
- The diagram below (Figure 8)shows the results Rutherford expected from the experiment.
Figure 8
Based on plum pudding model proposed by Thomson, Rutherford expected a beam of massive alpha particles would penetrate through gold atoms. He expected only a few deflections of the alpha particles.
The actual results observed by Rutherford are shown in Figure 9.
The results showed that some particles were deflected from their path; very few alpha particles were deflected by large angles or completely bounced back.
Figure 9
The Rutherford’s experiment showed that most of the alpha particles went through the gold foil while in fewer cases still the alpha particles were deflected through an angle greater than 90°.
Rutherford’s model of the atom
- Rutherford demonstrated that the plum pudding model proposed by Thomson was false.
- Based on some calculations, they showed that the diameter of the nucleus was about five orders of magnitude smaller than that of the atom.
- Using this model, Rutherford showed that the nucleus of an atom is of the order of 10-14 m across compared with the size of an atom which is of the order of 10-10 m.
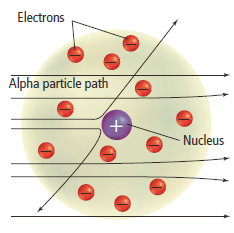
- The large angles of scattering for a very small number of particles led Rutherford to propose that the majority of the mass of the atom was concentrated in a minute positively charged region, around which the electrons in the atom circulated.
- When an alpha particle came very close to the nucleus, Rutherford reasoned, the electrostatic repulsion between the two would be sufficient to repel the alpha particle and so produce the large angle of scattering.
- Since the nucleus was small, this scattering would occur for only the few particles which approached the nucleus sufficiently closely.
- Rutherford’s nuclear atomic model is shown in the Figure 10.
Rutherford’s model of the atom conclusion
- Therefore they concluded that matter is mostly empty space, with the very lightweight electrons orbiting around an incredibly dense and positively charged nucleus.
- He also concluded that almost all of the positive charges were contained in a tiny, dense region in the centre of the atom, which he called the nucleus.
- The negatively charged electrons are held within the atom by their attraction to the positively charged nucleus.
Figure 10
The observation showed that some of the alpha particles were deflected backward implied that the positive charge in the atom must be confined to a highly dense region inside the atom known as the nucleus.
Summary of Rutherford’s experiment
| Observation | Conclusion |
| • Most alpha particles went straight through | • The atom contains large empty space |
| • Some particles were deflected. • Very few bounced back. | • Nucleus (positively charged center). • All mass of atom resides in the nucleus. • Size of nucleus is very small. • Electrons revolve in the empty space. |
| • Atom is electrically neutral | • Number of protons=Number of electrons |
The proton and the neutron
- By 1920, Rutherford had refined the concept of the nucleus and concluded that the nucleus contained positively charged particles called protons.
- A proton is a subatomic particle carrying a charge of 1+.
- In 1932, James Chadwick (1891–1974), showed that the nucleus also contained another subatomic neutral particle, called the neutron.
- A neutron is a subatomic particle that has a mass nearly equal to that of a proton, but it carries no electric charge.
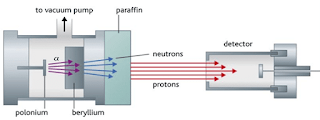
- Chadwick’s experiment consists of bombarding a beryllium plate with alpha particle. An uncharged radiation is produced on the opposite side of the sheet. Therefore, he used a solid material containing many hydrogen atoms (paraffin wax) in the path of this radiation caused protons to be knocked out of the wax. He showed that the unknown radiation must consist of uncharged particles with a mass similar to that of the proton.
Figure 11
The Atomic Theory
Structure of the Atom
- An atom is an electrically neutral, spherical entity composed of a positively charged central nucleus surrounded by one or more negatively charged electrons.
- All atoms are made up of the three fundamental subatomic particles—the electron, the proton, and the neutron.
- The electrons move rapidly within the available volume, held there by the attraction of the positively charged nucleus.
- An atom’s diameter (≈1×10-10 m) is about 20,000 times the diameter of its nucleus (≈5×10-15 m).
- The nucleus, which is composed of neutral neutrons and positively charged protons, contains all of an atom’s positive charge and more than 99.97% of its mass.
Figure 12
- An atomic nucleus consists of protons and neutrons (the only exception is the simplest hydrogen nucleus, which is a single proton).
- The proton (p+) has a positive charge, and the neutron (n0) has no charge; thus, the positive charge of the nucleus results from its protons.
- The magnitudes of the charges possessed by a proton and by an electron (e–) are equal, but the signs of the charges are opposite.
- Since an atom is electrically neutral, the number of protons in the nucleus of an atom must be exactly equal to the number of electrons.
Properties of Subatomic Particles
| |||||
Particle
|
Symbol
|
Location
|
Relative Electric Charge
|
Relative Mass
|
Actual Mass (g)
|
Electron
|
e–
|
In the space surrounding the nucleus
|
1-
|
9.11×10-28
| |
Proton
|
p
|
In the nucleus
|
1+
|
1
|
1.673×10-24
|
Neutron
|
n
|
In the nucleus
|
0
|
1
|
1.675×10-24
|
- The proton number of an atom is also known as the atomic number Z. This represents the number of protons in the nucleus.
- All carbon atoms (Z=6) have 6 protons, all oxygen atoms (Z=8) have 8 protons, and all uranium atoms (Z=92) have 92 protons. There are currently 117 known elements, of which 90 occur in nature and 27 have been synthesized by nuclear scientists.
Atomic number
atomic number = number of protons
= number of electrons
The atomic number of an atom equals its number of protons and its number of electrons.
- The mass number (or nucleon number) A is the number of protons plus the number of neutrons in the nucleus of an atom.
- Thus, a carbon atom with 6 protons and 6 neutrons in its nucleus has a mass number of 12, and a uranium atom with 92 protons and 146 neutrons in its nucleus has a mass number of 238.
Mass number
mass number = atomic number + number of neutrons
The mass number of an atom is the sum of its atomic number and its number of neutrons.
- An atom with Z protons and N neutrons in represented as shown in Figure 13. The atomic symbol X is based on the element Latin, Greek or English name.
- For example, C for carbon, S for sulfur, and Na for sodium (Latin natrium). Often written with the symbol are the atomic number (Z) as a left subscript and the mass number (A) as a left superscript.
Figure 13
- Since the mass number is the sum of protons and neutrons, the number of neutrons (N) equals the mass number minus the atomic number:
Number of neutrons = mass number – atomic number
N = A – Z
Examples
|
|
Z= 6 (atomic number)
N= 12-6 =6 (number of neutrons)
|
|
|
Z= 8 (atomic number)
N= 18-8= 8 (number of neutrons)
|
|
|
Z= 17 (atomic number)
N= 37-17 =18 (number of neutrons)
|
Isotopes
- Isotopes are atoms with the same proton number but different nucleon numbers. They have the same electron arrangement and , therefore, the same chemical properties
- For example, all carbon atoms (Z=6) have 6 protons and 6 electrons, but only 98.89% of naturally occurring carbon atoms have 6 neutrons (A=12). A small percentage (1.11%) have 7 neutrons (A=13), and even fewer (less than 0.01%) have 8 (A=14). These are carbon’s three naturally occurring isotopes: 12C,13C, and 14C.
- The chemical properties of an element are primarily determined by the number of electrons, so all isotopes of an element have nearly identical chemical behaviour, even though they have different masses.
Calculation of the atomic mass of chlorine
- To calculate the weighted average atomic mass of chlorine, you first need to calculate the mass contribution of each isotope.
Figure 14
Atomic Masses of the Elements
- The mass of an atom is measured relative to the mass of an atomic standard. The modern standard is the carbon-12 atom, whose mass is defined as exactly 12 atomic mass units. Thus, the atomic mass unit (amu) is 1/12th the mass of a carbon-12 atom.
- Based on this standard, the 1H atom has a mass of 1.008 amu; in other words, a 12C atom has almost 12 times the mass of an 1H atom.
- The other unit for atomic mass is dalton (Da).Therefore one 12C atom has a mass of 12 daltons (12 Da, or 12 amu).
- The atomic mass unit is a unit of relative mass, but it has an absolute mass of 1.66054×10-24 g.
Masses of Subatomic Particles
Mass of Subatomic Particles
| |
Particle Mass (amu)
|
Particle Mass (amu)
|
Electron
|
0.000549
|
Proton
|
1.007276
|
Neutron
|
1.008665
|
Mass Spectrometry
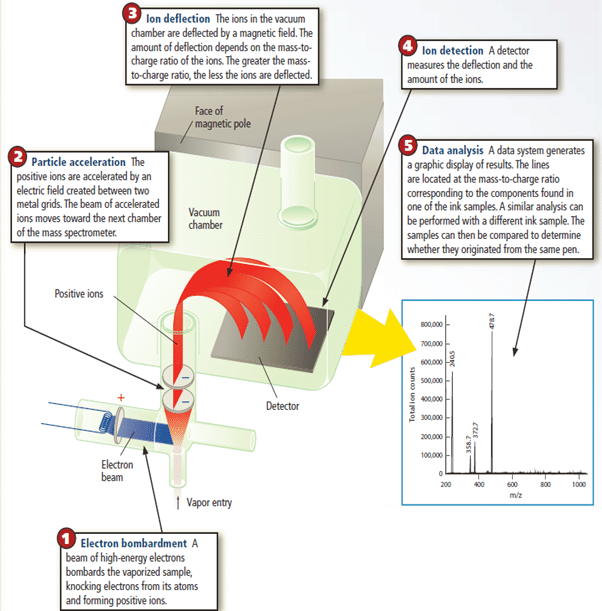
The isotopic makeup of an element is determined by mass spectrometry, a method for measuring the relative masses and abundances of atomic-scale particles and molecules very precisely. For example elemental neon atoms are bombarded by a high-energy electron beam as shown in Figure 15.

For example elemental neon atoms are bombarded by a high-energy electron beam as shown in Figure 15. As a result, one electron is knocked off each Ne atom, and each resulting particle has one positive charge. Thus, its mass/charge ratio (m/e) equals the mass of an Ne atom divided by 1+. The m/e values are measured to identify the masses of different isotopes of the element. The positively charged Ne particles are attracted toward a series of negatively charged plates with slits in them, and some of the particles pass through the slits into an evacuated tube exposed to a magnetic field. As the particles zoom through this region, they are deflected (their paths are bent) according to their m/e values: the lightest particles are deflected most and the heaviest particles least. At the end of the tube, the particles strike a detector, which records their relative positions and abundances as shown in the Figure 15. Mass spectrometry is now used to measure the mass of virtually any atom or molecule.
Figure 16
For instance, we use a mass spectrometer to measure the mass ratio of 28Si to 12C as illustrated below:
Mass of Si Atom / Mass of C Standard = 2.331411
Mass of Si atom= 28
Mass of C standard= 12
From this mass ratio, we find the isotopic mass of the 28Si atom, the relative mass of this silicon isotope:
Isotopic mass of Silicon-28 = measured mass ratio x mass of Carbon-12
= 2.331411 x 12 amu = 27.97693 amu
The mass spectrometer also gives the relative abundance as a percentage (or fraction) of each isotope in a sample of the element.
How the Mass Spectrometer Works
A mass spectrometer breaks the compounds in a sample of an unknown substance into smaller fragments. The fragments are then separated according to their masses, and the exact composition of the sample can be determined. Mass spectrometry is one of the most important techniques for studying unknown substances.
Figure 17
Unstable Nuclei and Radioactive Decay
Radioactivity
Nuclear reactions
- Substances that emitted radiation spontaneously in a process is called radioactivity.
- Radiations are rays and particles emitted by the radioactive material.
- A nuclear reaction involves a change in the nuclide.
- A nuclear reaction results in the formation of new kinds of atoms.
- Radioactive atoms produce radiation because their nuclei are unstable.
Radioactive decay
- Unstable nuclei disintegrate (break up) and lose energy by emitting radiation such as alpha, beta or gamma radiation. The disintegrated is radioactive decay.
- Unstable atoms disintegrate to form stable atoms, often of a different element.
Types of Radiation
- There are three different types of radiation based on their electric charge.
- An experiment was carried out to investigate the effect of electric fields and magnetic on radiation.
- By directing radiation from a radioactive source between two electrically charged plates, As shown in the Figure 17, radiation were deflected toward the negative plate, the positive plate, or not at all.
Alpha radiation
- Alpha particles having a positive charge deflected toward the negatively charged plate were named alpha radiation.
- An alpha particle contains two protons and two neutrons, and thus has a 2+ charge, which explains why alpha particles are attracted to the negatively charged plate as shown in Figure 17.
- An alpha particle is equivalent to a helium-4 nucleus and is represented by α.
- The alpha decay of radioactive radium-226 into radon-222 is shown below.
- The new element, radon (Rn), is created as a result of the alpha decay of the unstable radium-226 nucleus.
- The type of equation shown above is known as a nuclear equation. It shows the atomic numbers and mass numbers of the particles involved. The mass number is conserved in nuclear equations.
Beta radiation
- Beta particles having a negative charge deflected toward the positively charged plate were named beta radiation as shown in Figure 17.
- This radiation consists of fast-moving beta particles.
- Beta particle is an electron with a 1- charge and it represented by the symbol β or e– .
- An example of beta decay of carbon-14 into nitrogen-14 is shown below.
Figure 18
Gamma radiation
- Gamma rays are undeflected since they are electromagnetic and it possessed a high-energy radiation.
- Gamma rays have no mass and are denoted by the symbol γ.
- Gamma ray are neutral, gamma rays are not deflected by electric or magnetic fields.
- They usually accompany alpha and beta radiation, and they account for most of the energy lost during radioactive decays.
- An example gamma rays is shown below:
- Gamma rays are mass less; the emission of gamma rays by themselves cannot result in the formation of a new atom.
Characteristics of Radiation
| |||
Alpha
|
Beta
|
Gamma
| |
Symbol
|
α
|
β
|
γ
|
Mass (amu)
|
4
|
1/1840
|
0
|
Mass (kg)
|
6.65 x 10-27
|
9.11 x 10-31
|
0
|
Charge
|
2+
|
1-
|
0
|
Nuclear Stability – Atomic Structure Notes
- The primary factor in determining an atom’s stability is its ratio of neutrons to protons. Atoms that contain either too many or too few neutrons are unstable and lose energy through radioactive decay to form a stable nucleus.
- They emit alpha and beta particles and these emissions affect the neutron-to-proton ratio of the newly created nucleus.
- Radioactive atoms undergo enough radioactive decay to form stable, non-radioactive atoms.
Our other College Graduation – BSc Chemistry Notes are given below-
- B.Sc. Chemistry Notes - Atomic Structure
- University Chemistry Notes Thermodynamics
- Graduation Chemistry Notes - Electromagnetic spectrum UV and Visible Spectroscopy
- College Chemistry of Elements of First Transition Series