Solid State Chemistry
Solid: -
Matter which possesses rigidity having definite shape & volume is called solid.Types of solid: -
1. Crystalline Solid: - Eg:- Metals And Non metals
2. Amorphous Solid: - Eg:- Rubber, Glass, Plastic
Difference and Comparison between of characteristics
Crystalline Solid: -
Contain regular arrangement having short range
& as well as long range order.
Definite geometric shape,
Sharpe melting point, they have definite heat fusion, they undergo clean cleavage,
and they are true solid.
Amorphous Solid: -
Containing irregular arrangement having short range
order only, irregular shape, melting over range of temp, they do not have
definite heat of fusion they undergo an irregular cut they are pseudo solid or
super cooled solid.
Classification of crystalline solid:-
1. Ionic
Solid
2. Molecular Solid
3. Covalent of network
Solid
4. Metallic Solid
1. Ionic solid: -
In these crystalline solids, constituent particle
are positive or negative ion held together by columbic or electrostatic forces
of attraction.
Eg:-NaCl, Mgo
2. Molecular solids:-
These are of three types
1. Non
polar molecular solid: - in these the constituent particles are atoms of noble
gas or non polar molecules are held by London dispersion forces.
Eg:
- Ar, H2.
2. Polar
molecular solids: - In these solids constituent particles are held together by
dipole-dipole attraction forces.
Eg: - Hcl, SO2.
3. Hydrogen
bonded molecular solids: - in these types of molecular solids constituent
particles are molecule containing hydrogen linked to F, O and N held by
hydrogen bonding.
Eg:
- H2O (ice), NH3.
3. Covalent or Network Solids: -
Constituent particles are atoms held together by covalent bonds
Eg: - C (diamond), Sio2 (quartz)
4. Metallic solids: -
Constituent particles are positive ions in a sea of mobile electrons held together by metallic bonds.
Eg: - All metals and alloys.
Crystal Lattice: -
Regular arrangement of constituent particles in 3-D is called crystal lattice or space lattice.Unit cell: -
The smallest three dimensional portion of a complete space lattice which when repeated over and again in different direction produces the complete space lattice is called unit cell.Bravais lattice: -
The fourteen lattices corresponding to seven crystal system are known as Bravais Lattice.Coordination number: -
Tthe number of spheres touching to a particular sphere.No. of atoms per unit cell:-
(a) No.
of atoms in simple cubic lattice:- 8 atoms are at corner
(b) No.
of atoms in BCC:
8 atoms are at corner and one in
the body
8*1/8+1=2
(c) No.
of atoms in FCC:-
8 atoms are at corner and six atoms
are at faces (one on each face)
8*1/8+6*1/2=4
Close packing in crystals:-
(a) Close
packing in one dimension : - sphere are touching each other in a row. In this
arrangement coordination no. is 2
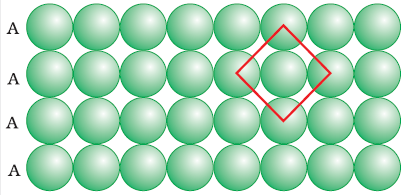
(b) Close packing in two dimension:- it is done by
two ways-
1. Square
close packing : - in this arrangement spheres of second row are exactly above
the first row.
2. Hexagonal
close packing: - in this arrangement the spheres of second row may be placed in
the depression of the first row.
(c) Close packing in three dimension
1. Three
dimensional close packing from two dimensional closed packed layer: - starting
from the square close packed layer the second layer and all further layers will
be built up such that they are horizontally as well as vertically aligned with
each other.
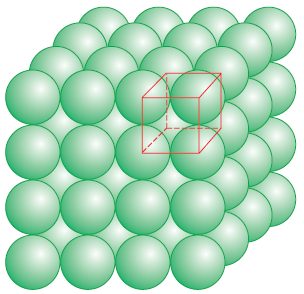 |
| Simple cubic lattice formed by A A A......... arrengment |
2. Three
dimensional packing from two dimensional hexagonal close packing:- when spheres
are arranged in hexagonal close packing in 2-Dthe two types of voids ‘a’&
‘b’ are formed.
‘a’
& ‘b’ voids are triangular when second layer is placed over the void of
first layer (only one void is full filled) in hexagonal close packing and these
layer form two voids
C
– Triangular and D – Octahedral.
 |
| A stack of two layers of close packed spheres and voids generated in them. T = Tetrahedral void; O = Octahedral void |
Packing efficiency: -
The percentage of the total space filled by the particles is
called packing efficiency.
Packing fraction: -
The fraction of the total space filled is called packing fraction.=>> Calculation of spaces occupied
1. in a simple cubic unit cell:-
Suppose
radius of sphere is = r
Edge
length of unit cell = a
As sphere are touching each other
a=2r
No.
of sphere per unit cell= 8*1/8=1
Volume of sphere = 4/3πr3
Volume of
cube =a3= (2r)3 = 8r3
Packing fraction
% occupied = 52.4%
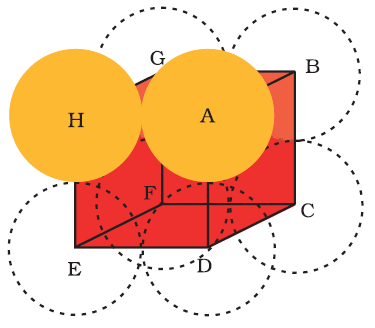
2.
in
face centered cubic structure (Cubic close packing):- spheres
of the face center touching the sphere of the corner,
Then,
AC = 4r
%
occupied = 74%
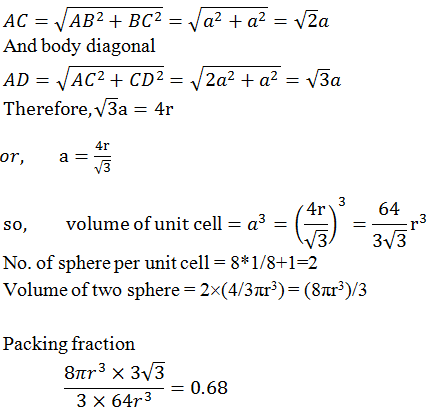
3. In body centred cubic structure: -
Sphere at the body centred touching the sphere of the corner.
Body diagonal, AD = 4r
%
occupied = 68%
=> Radius
(r) of the octahedral void =0.414R
=> Radius
(r) of the tetrahedral void = 0.225R
In CCP or HCC.
If there are N spheres in the packing.
No.
of octahedral voids = N
No. of Tetrahedral voids = 2N
Imperfection and defects in solids
Types of Defects:-
1. Stoichiometric
defects
2. Non-Stoichiometric
defects
3. Impurity
defects
1. Stoichiometric defects:-
In these defects the ratio of anion and cation remains same as the Stoichiometry of the substance not changed.
These are also classified as:
a. Vacancy
defects: - These arise when some sites of the crystal lattice are vacant, it
decrease density.
b. Interstitial
Defects: - When some constituent particles present in the interstitial side, it
increase the density of the crystal.
c. Schottky
Defects:- If in a ionic crystal +ve or –ve ions missing their lattice sites so
that electric neutrality is maintained
Ex: NaCl, KCl, CsCl, AgBr.
d. Frenkel
Defect: - If an ion missing their lattice site (causing vacancy or hole their)
and comes in the interstitial side, so maintain electric neutrality or Stoichiometry
of the crystal is called Frenkel defect.
Ex: ZnS, AgCl, AgBr, AgI.
2. Non-Stoichiometric Defect: -
In this defect ratio of cation & anion became different from the original ratio.
It
is of two types:
a. Metal
excess: - this may occur in two way-
I.
By anion vacancies: A –ve ion may missing
their lattice site and leaving a hole which occupied by an electron there by
maintaining electrical balance.
II.
By the presence of extra cation in the
interstitial site: - it is extra cation
(+ve ion) is occupying the interstitial site.
Anion
= -ve ion
Cation
= +ve ion
And
electric neutrality is maintained by an electron is present in another
interstitial site.
b. Metal
deficiency:- This defect shows variable vacancy and occurs usually due to the
missing of a cation from its lattice site and two monovalent cation is replaced
one divalent cation to maintain electric neutrality.
III.
Impurity defects :- These defects arises
when foreign atoms are present at the lattice site in place of host atoms or in
the interstitial sites.
Doping: -
The process of adding impurities to a crystalline substance so as to
change its properties is called doping.
Electrical properties of solids
1. Conductors:-
The solids which have conductivity in the range of 104 to 107
ohm-1m-1 are called conductors.
2. Insulators :-
The solids which have extremely low conductivity ranging between 10-20 to 10-10 ohm-1m-1 are called insulators.3. Semiconductors:-
The solids which have conductivity between the conductors and insulators, ranging from 10-6 to 104 ohm-1m-1 are called semiconductors.Band Theory
=> In
case of metals (conductors), the atomic orbital’s of the metal atom are so
close in energy that they overlap to form a large no. of molecular orbital’s
very close in energy. This set of molecular orbital’s is called a band. Two types of band
formed then electron can easily flow by gaining energy and conduct electricity.
=> If
gap between band is large electron can not jump from valance band to conduction
band, so these behave as insulator.
=> If
the gap is small then electron may jump and show some conductivity and act as
semiconductor.
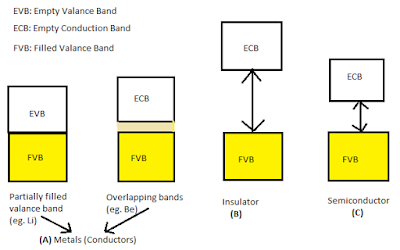 |
| Band Theory |
Magnetic properties of solids: -
1. Diamagnetic Substances: -
Substances which are weakly repelled by the external magnetic field are called diamagnetic substances.
Ex: TiO2, H2O, NaCl
2. Paramagnetic Substances: -
Substances which are attracted by the external magnetic field are called paramagnetic substances.
Ex: O2, Cu2+, Fe3+
3. Ferromagnetic Substances: -
Substances which show permanent magnetism even in the absence of magnetic field are called ferromagnetic substances.
Ex: Fe, Ni
4. Anti- Ferromagnetic Substances: -
Substances which are expected to posses’ paramagnetism and ferromagnetism on the basis of magnetic moments of the domains but actually they posses Zero magnetic moment are called anti- ferromagnetic substances.
Ex: MnO
5. Ferromagnetic Substances: -
Substances which are expected to posses large magnetism on the basis of magnetic moments of the domains but actually have small net magnetic moment are called ferromagnetic substances.
Ex: Fe3O4
 |
| Chemistry Notes Info for complete chemistry notes of school and college chemistry |
Online Education
by
Chemistry Notes Info
Solid State Chemistry MCQs
These are 20 selected MCQs from Solid State Chemistry which are very important and asked in various exams like AMU, KCET, MP CET, CBSE, Karnataka CEE, MP PMT, Kerala MEE, MP PET, AIIMS, BHU, IIT, EAMCET, NCERT, CPMT, AIEEE, CETMP, Pb PMT, AFMC.
MCQ-1. In Solids, the constituent particles may be :
Ions
Atoms
Molecules
Either of the above three
Atoms
Molecules
Either of the above three
MCQ-2. In a Crystal, atoms are located at position of the :
Maximum P.E.
Minimum P.E.
Infinite P.E.
Zero P.E.
Minimum P.E.
Infinite P.E.
Zero P.E.
MCQ-3. When identical spheres in the adjacent rows have a vertical as well as horizontal alignment in a way that each sphere has 4 nearest neighbours, This type of pattern is known as :
Cubic Close Packing
Body Centred Cubic Packing
Hexagonal Close Packing
Square Close Packing
Body Centred Cubic Packing
Hexagonal Close Packing
Square Close Packing








Chem - 9, 10, 11, 12, BSc, MSc APPs
ReplyDeletehttps://apkgk.com/com.appmakr.chemistrynotesinfo0